Describe Where a Hydrogen Bond Can Form Among Water Molecules
A covalent bond can be represented as a linkage between electron pair and two atoms. A covalent b ionic c hydrogen d atomic e electronic Answer.

Hydrogen Bonds In Water Article Khan Academy
Forces for the following chemical compounds HINT.
. It also allows plants to draw water from. In the Light Independent Process carbon dioxide from the atmosphere or water for aquaticmarine organisms is captured and modified by the addition of Hydrogen to form carbohydrates general formula of carbohydrates is CH 2 O n. Among many aspects of the progress in the development of the sustainable power package of the future catalysis or electrocatalysis has played a major role in overcoming the kinetic energy barriers for electrochemical reactions of water oxygen and hydrogen in water-splitting cells and fuel cells Fig.
Water is the chemical substance with chemical formula H 2 O. 1It is the role of catalysis in electrolysis water-splitting cells. A 11 mixture of CA and M molecules is prepared with Milli-Q water at a.
Hydrogen bond energies can span more than two orders of magnitude from about 02 to 458 kcalmol and the nature of hydrogen bond within this range varies ie its covalent electrostatic and dispersion. Electrocatalysis of the hydrogen evolution reaction HER is critical to the operation of water-alkali electrolyzers 16 in which hydrogen is the main product and chlor-alkali electrolyzers 5 6 in which it is a side productThese two technologies are highly energy-intensive and are known to account for 25 to 30 87600 to 92000 GWhyear of the total. Among many aspects of the progress in the development of the sustainable power package of the future catalysis or electrocatalysis has played a major role in overcoming the kinetic energy barriers for electrochemical reactions of water oxygen and hydrogen in water-splitting cells and fuel cells Fig.
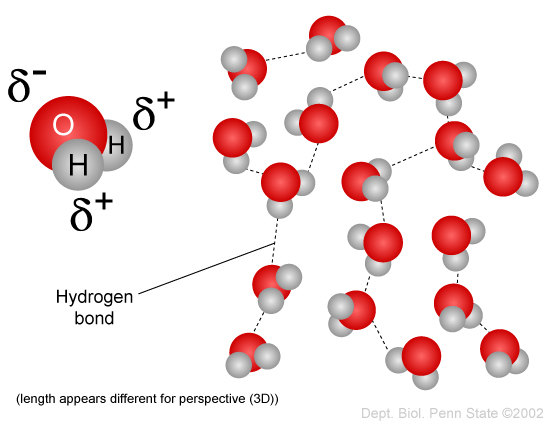
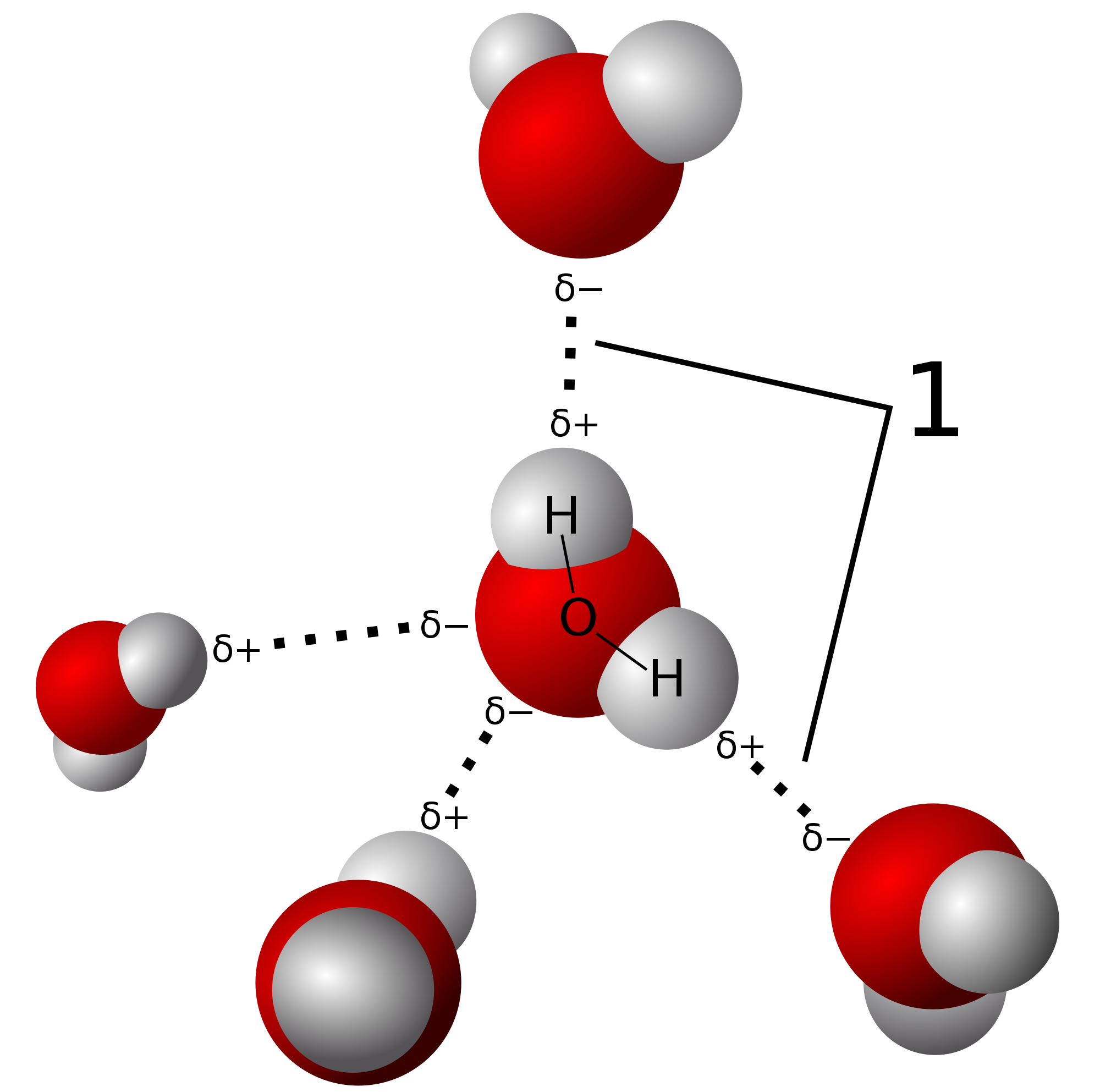
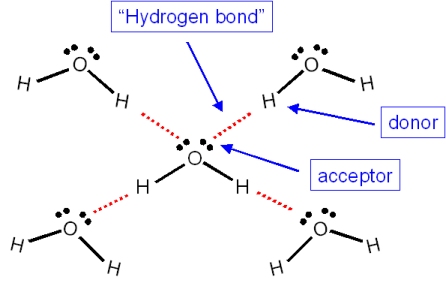
One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. It is the role of catalysis in electrolysis water-splitting cells. The energy for this comes from.
Among common liquids water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. This attractive force along with other intermolecular forces is one of the principal factors responsible for the occurrence of surface tension in liquid water. Describe isotopes of hydrogen.
Understand the structure of water and use the knowledge for explaining physical and chemical properties. Add various unknown molecules to oil and water and observe how the molecules sort themselves in response to interactions with the surrounding environment. SO 22 Explain how atoms form molecules and compounds and describe the nature of the various types of bonds that join them.
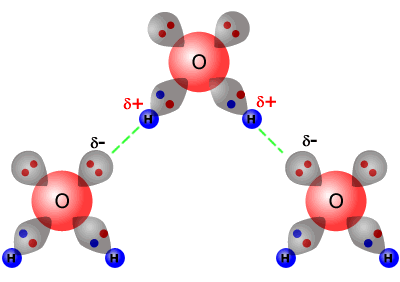
When two water molecules approach one another the slightly negatively charged oxygen atom of one forms a hydrogen bond with a slightly positively charged hydrogen atom in the other. Non-Bonding conceptual version Compare the electron distribution potential energy and forces of two interacting hydrogen atom which can bond with two helium atoms which dont. Acetone removers can be used to remove most unwanted household stains.
The hydrogen bond H-bond as shown in Fig. Dipole-Dipole Electrostatic Hydrogen or London Dispersion NaCl. The center carbon atom forms a double covalent bond with the oxygen atom and two single covalent bonds with the other two carbon atoms whereas all the 6 hydrogen atoms form single covalent bonds with the exterior carbon atoms.
Easy Study Objective 1. Individual CA molecules form aggregates on the MoS 2 surface at room temperature Supplementary Fig. To quantitatively study the interaction strengths among water molecules W.
Describe how an understanding of its properties can lead to the production of useful substances and new technologies. The cis-double bonds in the unsaturated fatty acids introduce a kink in their shape which makes it more difficult to pack their molecules together in a stable repeating array or crystalline lattice. From AFM phase.
Some insects like the one shown in even though. A steel needle carefully placed on water will float. The shapes of stearic and oleic acids.
SO 221 Describe how valence electrons form chemical bonds. Identify the types of. This can easily be observed in a.
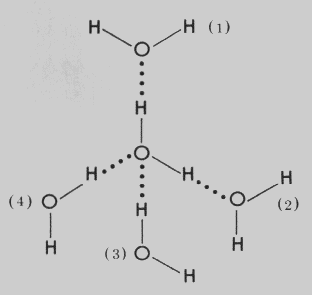
The trans-double bond isomer of oleic acid known as elaidic acid has a linear shape and a melting point of 45 ºC 32 ºC higher than its cis isomer. 11 requires that the distance between the Hw atom in the water molecule as the donator and the Ow atom in the water molecule as the acceptor be D HO 245 Å and the angle between the OO vector of two water molecules and the OH vector as the donator θ 30. SO 222 Distinguish among ionic covalent and.
Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. Hydrogen-bonded to the polar groups of PAAm molecular chains. As a result of this high surface tension the surface of water represents a relatively tough skin that can withstand considerable force without breaking.
Holds atoms together in an ionic covalent or metallic bond. Force is BETWEEN molecules or formula units. Different molecules such as hydrogen nitrogen chlorine water ammonia have a covalent bond and other ligands with sulfate amide silane carboxyl hydroxyl groups are covalently bound the MONPs and act as a linker between the biomolecules and NPs.
Explain how different elements combine with hydrogen to form ionic molecular and non-stoichiometric compounds. Acetone polish remover works by breaking down nail polish and. The incorporation of carbon dioxide into organic compounds is known as carbon fixation.
1 5 Hydrogen Bonds Biology Libretexts
Structure And Bonding 2 43 Hydrogen Bonding
Hydrogen Bond Mechanism Hydrogen Bond In Water Examples Videos

Showed Hydrogen Bonding Between Glucose And Water Molecules Download Scientific Diagram
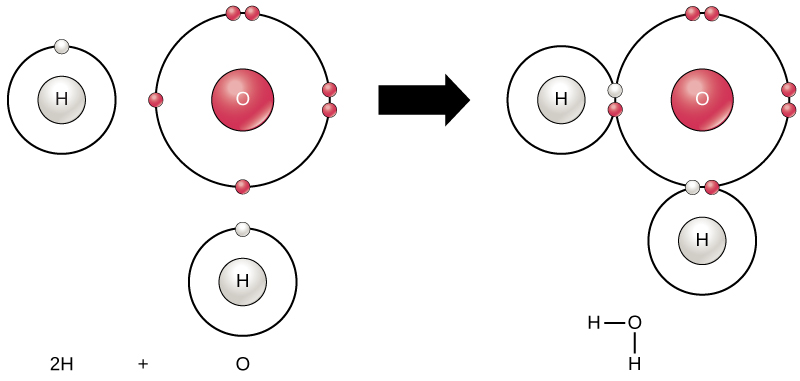
Chemical Reactions And Molecules Biology For Majors I
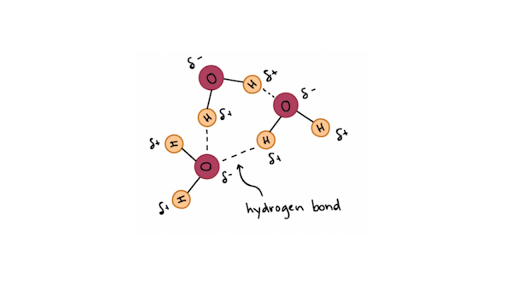
Hydrogen Bonds In Water Article Khan Academy

Hydrogen Bonding Chemistry For Non Majors

File 210 Hydrogen Bonds Between Water Molecules 01 Jpg Wikimedia Commons
Answer In General Chemistry For Jude 101935

Hydrogen Bonds What Are Hydrogen Bonds How Do Hydrogen Bonds Form Youtube

Hydrogen Bonding Chemistry For Non Majors

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
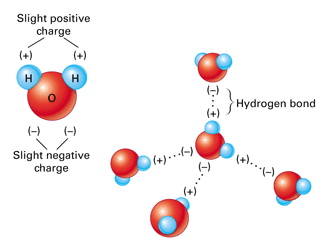
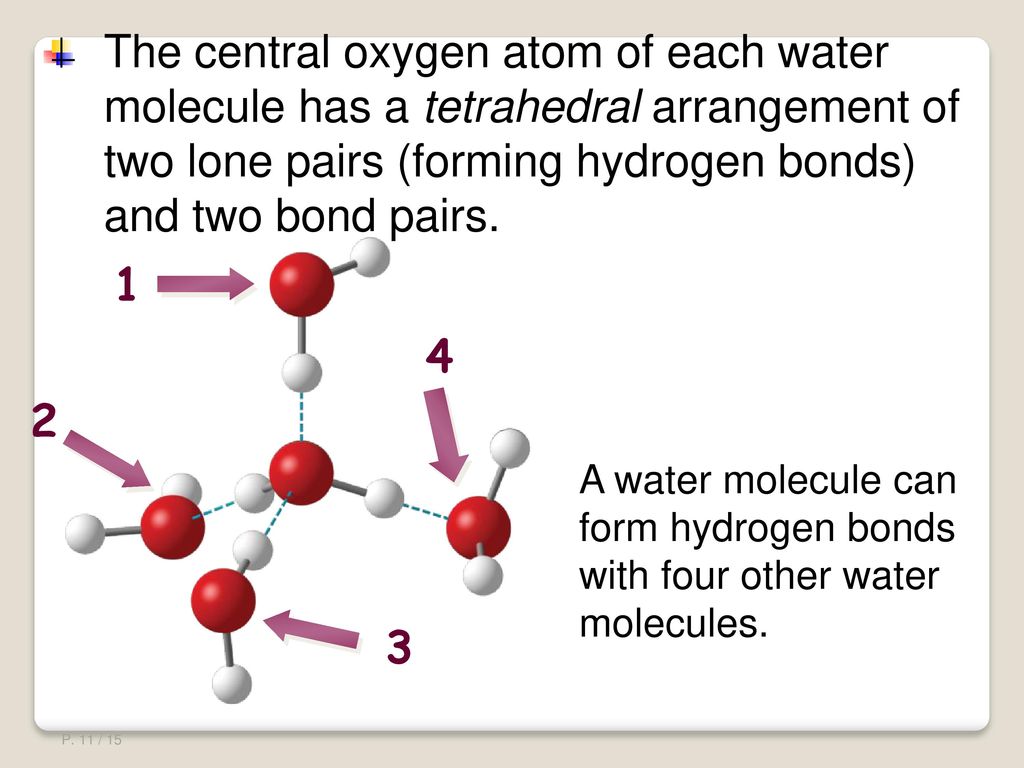

Comments
Post a Comment